Protein Chemistry
We have seen that proteins have many effects on food and cooking, from stabilizing foams and emulsions, to firming up gels. Knowing a little bit more detail about proteins will help in designing and modifying recipes, and in fixing things that go wrong.
Amino acids
Proteins are made up of amino acids. An amino acid has a central carbon, with a carboxyl group (COOH) at one end, and a amine group (NH2) at the other end. The simplest amino acid is glycine.
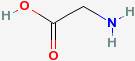
Notice that at the left end, there is a hydrogen attached to an oxygen. At the right end there is a hydrogen. Amino acids can join together by joining end to end, and losing a water molecule (the OH at the left and the H at the right).
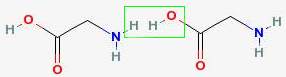
For example, two glycines can join to form diglycine. In the drawing below, the dotted green line shows where the two glycines have joined together.
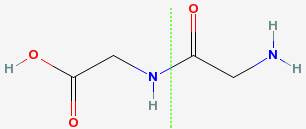
This kind of bond is called a peptide bond, and is very strong.
When only a few amino acids are joined together in this way, the molecule is called a polypeptide. Longer polypeptides are called proteins.
There are about 22 different amino acids found in the proteins that make up our bodies and the foods we eat. These amino acids differ from one another in one particular way – the things that are attached to the carbon right next to the nitrogen.
In glycine, there is just a hydrogen atom there (in the structural formulas used in this book, carbon atoms are assumed to be wherever a line changes direction, and hydrogen atoms are assumed to be attached to any carbons that do not have all four bonds filled up. Otherwise the drawings get very cluttered).
The sequence of amino acids in a protein gives it what we call the primary structure of the protein. You can think of the primary structure as a string of beads, with each bead being an amino acid.
In alanine, another simple amino acid, there is another carbon attached to that place, and it has three hydrogens attached to it (this is called a methyl group). Longer chains of atoms are attached in other amino acids. These chains can form their own bonds with one another, to create what is called the secondary structure. Two common secondary structures are the alpha helix, where the protein forms a coiled spring, or helix, and the beta sheet, where the strings of beads bond parallel to one another to form sheets. These forms are held together with hydrogen bonds, a weaker form of bonding where a hydrogen that is strongly held by a bond with something like carbon, is attracted to an electronegative atom like a nitrogen or an oxygen atom.
The tertiary structure of a protein is what we get when the protein folds into a three dimensional shape.
Soluble proteins often are globular or almost spherical in shape. Egg albumin is globular, and there is a whole class of proteins called globulins because of their shape. Insoluble proteins like collagen in connective tissue, elastin in tendons and arteries, and keratins in hair, hooves, and nails, are forms of fibrous protein. Some proteins are combined with other molecules to form conjugated proteins. In the nuclei of cells, nucleic acids combine with proteins to form nucleoproteins. Combined with just a little carbohydrate a protein is called a glycoprotein (from the word for sugar). Combined with more than about 4% carbohydrate, proteins are called mucoproteins. And combined with fat, they are lipoproteins.
These properties form what is called the quaternary structure of a protein. As you might guess from the name, there are four kinds of structure in proteins.
Denaturing proteins
In their natural state, proteins like egg albumin and milk casein are soluble in water. Most of their hydrogen bond forming parts are tucked inside the folded structure of the protein, making them unavailable for forming bonds with other molecules. They are all the same shape, so that they all have the same properties, and can form crystals.
There are several mechanisms that destroy these properties. Heat, acids, strong alkalis, alcohol, urea, salicylate, and ultraviolet light are among the more common ways that proteins become denatured.
A denatured protein unfolds, as many of the hydrogen bonds that preserve the three dimensional structure of the protein are broken. Instead of a uniform solution of molecules that are all the same shape, in a denatured protein, the molecules can take a staggering number of different shapes (on the order of 1020 different shapes, depending on the size of the protein molecule). Like snowflakes, few if any of the molecules will have the same shape, and they will no longer form regular crystals.
The unfolded molecules also have more bond forming areas exposed on the outside, and they form bonds with one another, and coagulate. They become insoluble in water.
We have seen surface effects cause proteins to denature. When we beat egg whites, or whip cream, the proteins unfold as their hydrophobic parts rearrange to avoid water in favor of air or fat. The infolded proteins can then bond to one another to form stabilizing protein films that keep the new forms in the desired shape.
In cooking, we control the denaturing of proteins in several ways. We can control the temperature, we can control the acidity, we can use copper bowls to beat the egg white and catalyze the formation of disulfide bonds in the proteins, and we can control the fat or air content when we beat the proteins.
For example, when beating egg whites, it is important to keep fats out of the egg whites. A little bit of oil or egg yolk can prevent the foam from stabilizing, as the air has competition with fat for the hydrophobic parts of the molecule.
Because proteins have both acidic parts and basic parts, the acidity of the solution they are in changes their behavior. Acids release protons (hydrogen nuclei) and bases accept protons. In an acidic solution, the basic parts of the protein accept protons from the acidic solution and become positively charged. The positive charges repel one another, and the protein molecules are less likely to combine with one another.
In a basic solution, the acidic parts of the protein lose a proton, and become negatively charged. This also results in repulsion between the protein molecules, and combination is reduced.
Charged areas of the protein interact with water molecules, because water is a polar molecule, with one end negative and one end positive. These charged ends are attracted to opposite charges on the protein.
Milk
The main proteins in milk (nearly 80%) are casein proteins. Caseins have a lot of the amino acid proline, which causes proteins to bend wherever there is a proline. This makes the proteins unlikely to stack into regular orderly secondary structures. In addition, caseins have no disulfide bonds, and so they have little tertiary structure. This means that the hydrophobic parts of the molecule are open and exposed (not tucked inside a ball).
All of this combines to give caseins interesting properties. The hydrophobic parts end up migrating together, and the hydrophilic parts arrange themselves to the outside, facing the water. These tiny hairy balls of protein are called micelles.
Caseins bind together with calcium and phosphorus. Without the caseins, calcium phosphate would not be soluble. As a nutrient for the young mammal that needs to build bones, this is a useful property. In the basic solution of milk, the hydrophilic parts of caseins become negatively charged, and they repel. This allows milk to stay liquid. But caseins clot in the stomach, due to acids that counteract the negative charges, and enzymes that cut the proteins into smaller pieces. This clotting makes the proteins stay longer in the stomach, releasing amino acids slowly, which aids digestion and absorption of the protein.
In the stomach of young mammals, enzymes cut off part of the water soluble casein (kappa-casein) that has the negative charges that keep the micelles apart. In making cheese, these enzymes, extracted from the stomachs of young calves, are used to make the caseins clot together into a solid.
Eggs
The proteins in eggs largely determine the characteristics of foods that contain eggs. Understanding the different properties of these proteins can be helpful when cooking, or when creating new dishes.
When you crack an egg into a pan, you can immediately see three parts. There is the yolk, a thin watery white, and a thick gelled white.
Egg white contains several mucoproteins, where the protein is attached to carbohydrates. In the egg, these serve as nutrients for the growing embryo, and as support and protection.
Over half of the protein in egg white is of one type – ovalbumin. It denatures at 176° Fahrenheit, forming the solid white mass we see at breakfast.
The next most prevalent protein in egg white is ovotransferin (also called conalbumin), which makes up about 12% of the proteins in the white of the egg. It denatures at a lower temperature, about 145°.
The third most prevalent protein is ovomucoid, at 11%. It is found close to the yolk, mixed with other proteins, thickening them.
When you crack an egg into the frying pan, the thin part of the egg white has less ovomucin, and the thick part of the white has 2 to 4 times as much. Ovomucin is the main gelling agent in egg white.
The first protein to denature when heating an egg white is the ovotransferin. As it unfolds it binds not only to other unfolded ovotransferin molecules, but to other proteins that are not yet denatured. The ovomucoid and ovomucin molecules, which do not coagulate with heat by themselves, can thus be incorporated into a strong gel with the ovotransferin and ovalbumin.
The remaining proteins make up less than a quarter of the protein in egg white, but some of them bear mentioning here. Avidin makes up a very small portion of the egg white (less than a tenth of a percent) but it binds very tightly to the essential nutrient biotin, making the biotin unavailable as a food source. This effect is destroyed when the protein is denatured by heat or beating, but can be a problem in a diet that contains a lot of raw egg white.
Meat
Raw meat is tough because each tiny packet of muscle fibers is surrounded by a tough sheet of connective tissue. This is the same tissue that makes gelatin when it is boiled.
When meat is cooked, the tough connective tissue denatures, and becomes soft gelatin. The proteins in the muscle fibers also denature. Enzymes in the tissue no longer function when they are denatured, so cooked meat will keep longer than raw meat.
If the meat is overcooked, the water in the fiber bundles boils, and the gelatin bag holding them in bursts, and the meat dries out. At high temperatures, the proteins also undergo further denaturing and cross linking, making the meat tough again. Crisp bacon is an excellent example of this. A thick juicy steak would be inedible if cooked to the hardness of bacon.
Enzymes
Enzymes in foods are often a problem for food storage. As cells break open, the enzymes inside them can leak out, and react with other parts of the food, causing brown soft spots in fruits and vegetables, and making meats smell and taste bad. The damaged parts also invite decay microorganisms.
Denaturing the enzymes can help to preserve the food. The heat of cooking is one familiar way to denature enzymes, but proteins can also be denatured by acids, strong alkalis, desiccation, or salt.
Shortening
When wheat flour and water are mixed and kneaded, sheets of gluten are formed. With further kneading, these sheets stick together into larger and larger sheets.
But if oils or fats are added, the hydrophobic amino acids in the gluten attach to the fat, and are not available to form bonds with other gluten molecules. This changes the nature of the dough, making it more tender, and less able to trap bubbles of leavening gasses.
The result is a more cake-like, less bread-like structure.
Glutamate
One particular amino acid has a strong effect on the taste of foods. That is glutamic acid, and salts of it called glutamates.
Besides being an abundant neurotransmitter in the brain, glutamate activates sensors on the tongue that detect savory protein-rich foods. Meats, poultry, fish, cheese, and soy sauce are rich sources of glutamate. The commercial form of pure glutamate is monosodium glutamate, and is an additive in many foods.
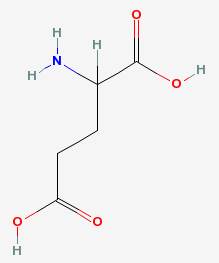 Glutamic acid
Glutamic acid
Cheese
Cheese is made from milk that has been curdled by the addition of acids and an enzyme from the stomach of calves called rennet. The acid can be from almost any food source, but for the most part it is produced by bacteria that convert the milk sugar lactose into lactic acid. Yogurt is also produced this way.
Cheese can be made without rennet, but the enzyme makes the curds stronger and more rubbery. Rennet allows the milk to curdle with less acid, which in turn allows flavor producing bacteria to colonize the curd. Cheeses made with rennet will melt easily, while cheeses made with acid alone remain intact at high temperatures.
The curds are salted, and moisture pressed out, so the product will not be as easily attacked by bacteria as raw milk would. Thus cheese making is a way of preserving milk.
