Oils and Fats
Oils and fats
The uses and properties of oils and fats are familiar to everyone who eats. But what gives them those properties is not so commonly known. We have already discussed why oil and water do not mix. But we can list some other properties of oils and fats, and then see if we can understand why they behave the way they do.
· Oils and fats float on water.
· Oils and fats have more calories than sugar and protein.
· Oils are more viscous than water.
· Fats soften slowly instead of melting at one temperature like water does.
· Oils don’t evaporate as quickly as water.
A water molecule is polar, with one side positively charged and the other side negatively charged, and tends to link up with other water molecules. The positive end is attracted to the negative end of another molecule, and they draw close together due to the attraction. This makes water dense.
Oils and fats are not polar. They don’t have positive and negative parts that attract one another. Instead, they are attracted by much weaker but similar forces that arise because the electrons in the molecules are always moving. When electrons move to one side of a molecule, a temporary state exists where one side is more negative than the other. Electrons in a nearby molecule are attracted to the more positive side, and for a very short time the two molecules are synchronized, with their electrons on the same side, so they behave as if they were polar. But this attraction is very weak, and small amounts of heat energy break them, and they form and break continuously.
So oils and fat molecules are only weakly attracted to one another, and do not pack together as tightly as water molecules do. So oil is less dense than water, and floats.
We get energy from food by burning it (combining it with oxygen). If we look at a sugar molecule, we see that it already contains oxygen. It is already partially burned:
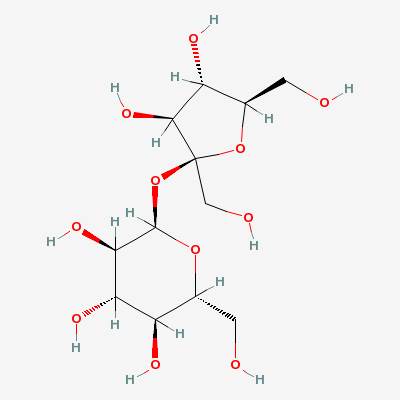
1 oxygen atoms, 22 hydrogen atoms, and 12 carbon atoms. Because there are twice as many hydrogen atoms as oxygen atoms, and H2O is water, these molecules are called carbohydrates – carbon plus water.
Fat molecules are mostly just carbon and hydrogen. A typical triglyceride only has six oxygen atoms, and might have over 50 carbons and over 100 hydrogens. Because less of the molecule is already burned, fats contain more fuel than carbohydrates.
A typical triglyceride found in fats is tristearin:
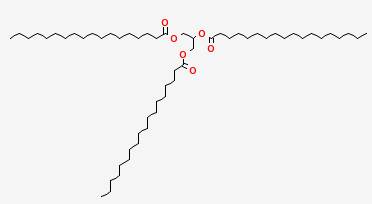
Three long chains of carbon and hydrogen are attached to the central glycerin molecule. These long chains rub against and tangle with other long chains on nearby tristearin molecules, and make it more difficult for the molecules to move about than is the case for water molecules. The result is a thicker liquid, with a higher melting point than water. In fact, tristearin is a solid at room temperature, melting at 161° F (72° C). But it is still less dense than water, and floats.
The long chains in fats like tristearin can line up together, so the attractions between the molecules are stronger than if they only touched at one or two points. This attraction raises the melting point. There are tryglyceride molecules where the long chains have kinks in them, so they don’t line up easily. These have lower melting points. Oils have more of these kinked molecules than fats do, so they are liquid at room temperature.
Because the molecules are so big, it takes more energy to get them to leave the liquid state and become a gas. Oils and fats do not evaporate as easily as small molecules like water.
Most fats are a mixture of many different types of tryglyceride molecules. In pure form, each molecule would make a liquid that has a distinct sharp melting point. But when many different types are all mixed together, the substance softens slowly over a range of temperatures, instead of melting at one temperature. When ice melts, it does not soften slowly. Instead, we have two distinct phases. The solid ice is coated with liquid water, and the two are at different temperatures. But fats slowly become soft and can be spread or mixed with other ingredients before they are completely melted.
Saturated fats
Carbon atoms can bond to four other atoms. In the long chains in the triglyceride molecules, each carbon is attached to the next carbon atom by either a single bond, or by a double bond. A double bond uses up two of the four available bonds, so each double bonded carbon can only hold onto one hydrogen instead of two. If the carbons are attached with only a single bond, each carbon has room to hold onto two hydrogen atoms. We say fats that have as much hydrogen as possible are saturated with hydrogen, and are saturated fats.
Tristearin is an example of a saturated fat. Saturated fats have chains that are not kinked. They can lie together closely like straight hair.
Monounsaturated fats
A double bond in a chain introduces a kink in the molecule. Because there are two bonds, the molecule is constrained in its motion, like a door with two hinges. The kink makes it harder for the molecules to line up together, so the attractive forces are less.
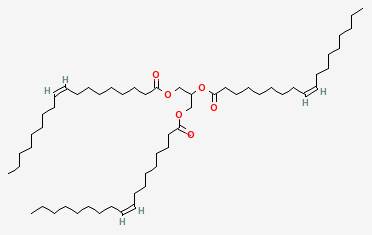
When there is only one double bond in the long chain, we say the fat is mono-unsaturated. A typical monounsaturated fat is triolein. It stays liquid at temperatures below freezing (22°F, -5°C). The oil in macadamia nuts is almost 80% monunsaturated, and olive oil is 75% monounsaturated.
Triolein:
Polyunsaturated fats
When more than one bond in the chain is double, we get polyunsaturated fat. As the number of double bonds increases, the melting point of the fat begins to increase again. But unlike saturated fats, where the high melting point is caused by the straight chains stacking up next to one another, the double bonds of polyunsaturated fats get tangled together, which also increases the melting point.
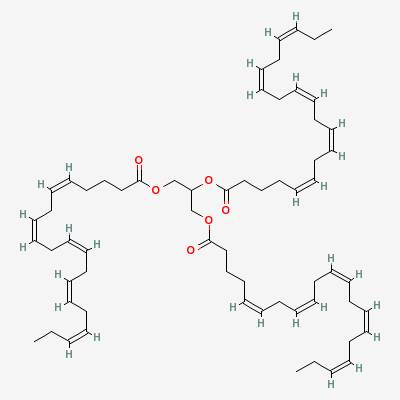
An example of a polyunsaturated fat has the conveniently memorable name of trieicosapentaenoin. We’ll call it triEPA for short. In triEPA, each of the three chains has five double bonds. The name comes from the fact that there are 20 carbons in the chain (the Greek word for 20 is eicosa), and five double bonds (the Greek for five is penta).
Trieicosapentaenoin is found in fish oils. The fish don’t make it, they get it from the algae they eat. The three chains are made up of eicosapentaenoic acid (EPA for short), which the human body converts into many essential substances called eicosanoids.
EPA is also converted into docosahexanoic acid (DHA for short), the major fatty acid in sperm, and the precursor molecule to many important hormones.
Trieicosapentaenoin:
Omega 3 and Omega 6 fats
If we look at the ends of the three chains in triEPA, we see that the last carbon in the last double bond is attached to a very short (2 carbon) chain at the end. The tail of the chain is called the omega end, named for the last letter in the Greek alphabet. The third carbon from the end is the one with the double bond.
Such a fat is called an omega-3 fat. More commonly, each of the three chains is said to consist of an omega 3 fatty acid.
In humans, omega-3 and omega-6 fatty acids are used to create many hormones that control the immune system and other functions. The enzymes that perform the conversions are the same for both types of fatty acids. But the omega-6 fatty acids can hog these enzymes, so that more omega-6 hormones are produced than omega-3 hormones. This can cause inflammation, arthritis, heart attacks, strokes, and depression. The typical western diet contains ten times as much omega-6 fat as omega-3 fat, resulting in hormonal imbalances that contribute to all of those diseases.
An example of an omega-6 fat is triarachidonin:
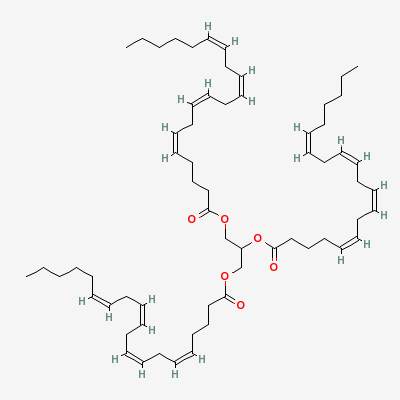
The drugs called COX-1 and COX-2 inhibitors used to treat pain and inflammation (aspirin, ibuprofen, naproxen) work by preventing arachidonic acid from being converted into inflammatory compounds.
Trans fats
The fats we have looked at so far have both hydrogens at each double bond on the same side of the chain. We say the hydrogens are in the cis position, from the Greek word for “on the same side”. When the hydrogens are both on the same side, the chain kinks.
But there is another position they can be in, where one hydrogen is on one side, and the other is on the opposite side. We call this the trans position.
The chains in trans fats are straight, like those of saturated fats. Trans fats have high melting points, and act a lot like saturated fats.
Trans fats form when unsaturated fats are heated. As the heat makes the molecules shake and vibrate, some of the bonds get changed from the cis to the trans position. As the fat continues to cook, the bonds may change back and forth many times, and the result is a fat that has about half of the bonds in the cis and half in the trans positions.
In the making of vegetable shortening, vegetable oils containing unsaturated fats are heated with a catalyst and some hydrogen, so that some of the double bonds gain a hydrogen and convert to saturated single bonds. But the process requires heat, and the heating produces undesirable trans bonds.
Trans fats have been shown to increase the risk of coronary heart disease by raising the levels of bad LDL cholesterol and lowering the levels of good HDL cholesterol.
Shortening was invented as a way to make solid fat from cheap vegetable oils, which could be substituted for the more expensive lard in cooking. It was only recently that the health problems associated with trans fats were discovered. Originally, vegetable shortening and margarine were sold as healthier alternatives to lard and butter.
These days, shortening is made by continuing to saturate the oils, so that there are almost no double bonds left, and then mixing the product with unsaturated oils until there is less than a gram of trans fat in a tablespoon of shortening. This allows the manufacturer to claim that there are zero grams of trans fat in the shortening.
Since there are 12.8 grams in a tablespoon of shortening, there can be 6% trans fat left in the shortening and still qualify for the label “zero grams” of trans fat. In other words, there can be 16 grams of trans fat in a cup of shortening that claims to have zero grams.
Compare the structure of triolein shown earlier with its trans version shown below:
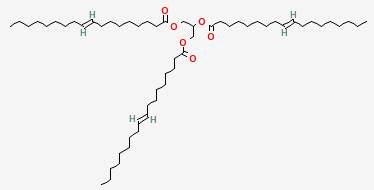
Here the chains are not kinked. They are shown as being straight, but actually the long chains can bend like spaghetti, except where there are double bonds. There, the movement is constrained to a hinge-like motion, due to the carbons being linked in two places instead of just one.
Saturated fats have the most flexible chains, since all the bonds are single bonds. They can pack together tightly in many configurations, and so they have the highest melting points. Trans fats are almost as good at packing, and have melting points in between that of similar saturated and cis-unsaturated fats. In the kinked cis-unsaturated fats like triolein, the kinks get in the way of packing, and the melting point is considerably lower.
