Oxidation and reduction
In acids and bases, we saw that protons (hydrogen ions) could be transferred between molecules. Those protons are positively charged subatomic particles. The most familiar negatively charged subatomic particle is the electron. When electrons are transferred between molecules, we call the process oxidation.
We say that something is oxidized when it loses electrons, and something is reduced when it gains electrons. It may seem backwards to think of gaining electrons as being ‘reduced’, but the name is a historical accident, because Benjamin Franklin chose the names for positive and negative charges before the discovery of the electron. Since electrons have negative charges, gaining an electron makes the oxidation number smaller, because we are adding a negative number.
As a proton donor is called an acid, an electron donor is an reducing agent. An electron acceptor is a oxidizing agent. (This simplified picture of oxidation is enough for our purposes in the kitchen, but as with most things, it can get more complicated as you drill down deeper.)
When you burn a candle, the oxidizing agent is the oxygen in the air, which steals electrons from the carbon and hydrogen in the candle wax. The result is oxidized carbon and hydrogen, known as carbon dioxide and water, respectively.
To cook on a gas stove, you don’t need to know much more than that. But much of the oxidation in the kitchen happens more slowly. Iron rusts, apple slices brown, we breathe in oxygen and breathe out carbon dioxide and water vapor, oils and fats get rancid, and wine turns into vinegar.
Apples, avocados, and lemon juice
Plants produce an enzyme called polyphenol oxidase. An oxidase is any enzyme that oxidizes something. The thing being oxidized in this case is a polyphenol.
We have seen polyphenols earlier, we just didn’t group them together under that name. Vanillin is a polyphenol. Tannins are polyphenols. Anthocyanins are polyphenols. Polyphenols from wine (resveratrol) and green tea (EGCG, or epigallocatechin gallate) are sold in health food stores. They are part of a larger class of compounds called antioxidants, because they are good at combining with harmful oxidizers. Their health benefits probably come from their other biological activities, however, as there is doubt that they act as antioxidants in the human body when consumed.
There are colorless polyphenols in apples, and there is polyphenol oxidase in apples. These two molecules are normally kept apart, but when you slice into an apple, you break open cell walls, and allow the two to mix and react.
As the enzyme combines the polyphenols with oxygen from the air, it produces quinones, which then combine (polymerize) to make dark colored molecules called melanins.
In an apple, one of the polyphenols is chlorogenic acid:
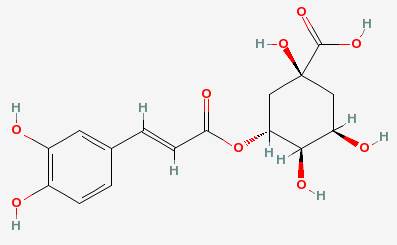
The phenol part is the hexagon on the left, with the two hydroxyl groups attached to it. The polyphenol oxidase enzyme removes the hydrogens from those to make a molecule called a quinone. A series of reactions then converts the quinone into melanin:
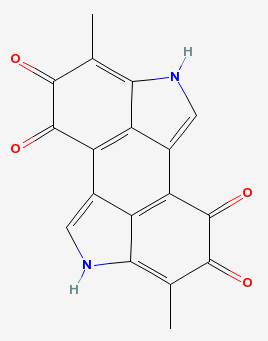
Melanins are the molecules responsible for the brown in brown hair, the red in red hair, the brown of a suntan and of freckles. They are found in the ink of squids and octopi. And in the cut apple, they make the unappetizing brown stains on the surface, or brown bruises below the surface.
There are a number of ways to interfere with these complex chemical reactions, and thus prevent the fruit from browning. Enzymes are proteins, and they can be denatured with heat until they no longer function. Blanching fruit by dipping it in boiling water will prevent browning.
The polyphenol oxidase enzyme also only works in a limited range of acidity. Making the juice on the apple slice too acidic or too basic will prevent the enzyme from performing its function. A little citric acid sprinkled on the cut will do the trick.
Enzymes work more slowly, or not at all, when the temperature is reduced. Refrigeration or freezing will slow or prevent browning.
Like most enzymes, polyphenol oxidase needs water in order to work. Dehydration will prevent browning. However, dehydration takes time, and the browning reaction is quick, so while the dehydration is happening, we can use yet another trick, and simply prevent oxygen from the air from getting to the cut fruit. We could also freeze-dry the apple slices.
We could denature the enzyme with X-rays (irradiation) or electron beams. High pressure can also denature the enzyme.
Or we could remove the oxygen by using a more powerful reducing agent than the polyphenols. Ascorbic acid (Vitamin C) can be sprinkled on the cut fruit. It will combine with the oxygen and make it unavailable to the enzyme. It can also make the juice more acidic at the same time, so it has a double action. And, of course, you have just improved the nutrition of the fruit by adding a vitamin.
Other reducing agents used are sulfites, which are often added to cut fruit before drying in the sun.
Vinegar from wine
Vinegar is made from wine that has been left open to the air. The ethanol in the wine oxidizes into acetic acid. The oxygen attaches to the central carbon atom:
But the ethanol doesn’t simply “burn” or “rust” into acetic acid. The transformation is done with enzymes, and the enzymes are produced by a bacterium called Acetobacter aceti.
The bacterium, like most organisms, has a preferred environment. It grows best at around 80° F to 85° F, with no more than 15% alcohol, with 9% to 12% being optimal. It also needs oxygen, unlike the yeast that made the alcohol in the wine.
As the bacteria grow, they form a mat of cellulose and other polysaccharides at the bottom of the wine. This mat is called “mother of vinegar”, because adding it to wine provides the starter culture of bacteria to make the vinegar.
Vinegars can be made from any fermented alcoholic beverage, and often are. Malt vinegar is made from ale or beer, for example. Fruit vinegars, rice vinegar, honey vinegar, and vinegar from sugar cane are popular in many places around the globe.
Oxidation of oils and fats
Oils and fats react with oxygen in ways that are sometimes beneficial, and sometimes undesirable. When cooking oils react with oxygen, they can form bad tasting compounds, and we call them rancid. But when oils react with oxygen and polymerize into tough insoluble films, we use them in paints and coatings for wood products.
How oxygen forms molecular bonds
An oxygen atom has six electrons in its outer electron shell, but eight electrons can fit in that shell. If two more electrons can be borrowed from another atom to form a molecule, the molecule will have a lower energy than the two separate atoms had, resulting in a strong covalent bond. To break the bond would require adding the energy back. In the drawing below, two oxygen atoms are shown, with the empty slots for two more electrons shown in gray.
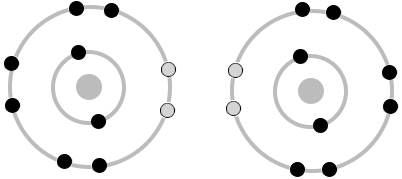
Two oxygen atoms can form two covalent bonds with one another. This lets two oxygen atoms fit together, so that they share the outer electron shell that has eight slots for electrons. The shared electrons fall into the holes in the outer shell and fill them up, locking the two atoms together. This is called a double bond.
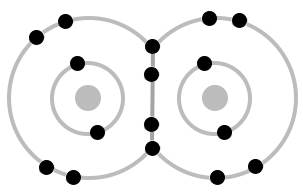
Oxygen molecules can also form with a single bond between the atoms. This leaves one lone electron on each oxygen atom.
There is a rule in quantum mechanics called the Pauli exclusion principle, which accounts for why atoms take up space. It limits how many electrons can be in the different orbitals of the atom. If there is no room in a low energy orbital, the electron can’t fall into a lower energy state, and stays in the higher energy orbital. Two electrons can occupy the same orbital if they have opposite spins.
Free radicals
When there is only a single bond between two oxygen atoms, the two lone electrons in each oxygen atom are not paired with an electron of the opposite spin. Such unpaired electrons are called radicals (sometimes free radicals), and are very reactive, since another electron from another atom can fall into the empty slot to pair with the lone electron, and form a covalent bond.
Since the oxygen molecule with a single bond has two unpaired electrons, it is called a diradical.
The double bond between the two atoms in an oxygen molecule was broken into a single bond and two unpaired electrons. Bonds in other molecules can also be broken this way to form radicals. Single bonds between two atoms can be broken such that each remaining part has an unpaired free electron, so that two radicals are formed. They can escape to react with other molecules, or they can join back together, releasing energy.
A quantum view of covalent bonding
Quantum mechanics deals with uncertainty and probability. Atomic orbitals are solutions to the Schrödinger wave equation that show where electrons are most likely to be located. Atomic orbitals can add together to form molecular orbitals. Between any two atoms in a molecule, where the waves are in phase and reinforce one another, the electron density (where electrons are most likely to be found) is greatest. This area of negative charge attracts the positively charged nuclei of the two atoms, and holds them together. This is why a covalent bond binds two atoms to one another.
Radical chain reactions
Oils and fats oxidize by a process called a radical chain reaction. A photon of light can start the reaction by breaking a single bond between a hydrogen and a carbon in a fatty acid in the oil.
This results in a radical, where the carbon now has an unpaired electron.
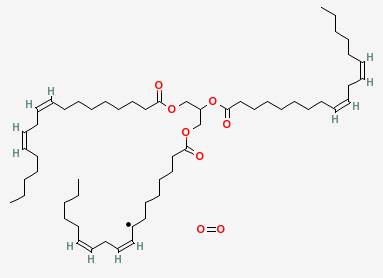
The unpaired electron then reacts with an oxygen molecule. It breaks the oxygen double bond, and pairs with one of the resulting unpaired electrons, leaving a peroxide radical attached where the hydrogen had been.
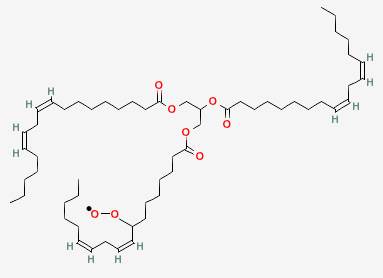
The remaining unpaired electron in the peroxide radical then steals a hydrogen from another linolenin molecule, which continues the chain reaction by bringing us back to where we started.
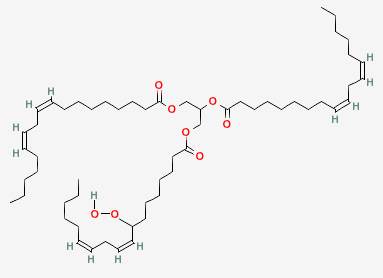
This chain reaction can go on for a long time, causing many of the oil molecules to be oxidized and become rancid. This is one reason why radicals are so damaging.
Eventually the radical meets up with another radical, and they cancel each other out by sharing electrons to fill out each other’s pair. At this point the chain reaction stops.
So, to keep oils from going rancid, we can either prevent the chain reaction from starting, by keeping the oils in the dark so no photons of light can create radicals, or we can slow down the reactions by keeping the oil cold in the refrigerator, or we can stop the reactions by using antioxidants to bind with the radicals before they can do too much damage.
Antioxidants
Some molecules have stable radicals. These are unpaired electrons that are either tucked in a crevice in the molecule where they aren’t readily available, or that are next to a conjugated ring that stabilizes the unpaired electron and make it less reactive. These molecules can stop the chain reaction by pairing up with the radical that would otherwise react with the oil.
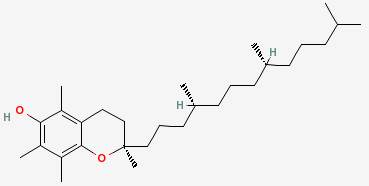
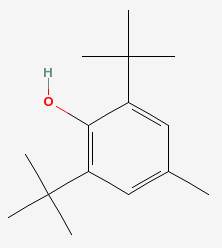
There are many such molecules, called antioxidants, and the drawing above shows alpha tocopherol, or vitamin E, which is one of them. Another one is butylated hydroxytoluene, or BHT.
We have seen other molecules earlier with conjugated rings (depicted by hexagons with alternating double and single bonds). Anthocyanins are good antioxidants. In fact, many of the colored parts of plants owe their color to antioxidants.
