Boiling, Freezing, and Pressure
It is fairly simple to lower the boiling point of water. We just lower the pressure of the air pushing down on the water, and this makes it easier for the water molecules to escape into the air. In fact, we can make water boil at room temperature if we lower the pressure enough.
An extreme version of this is the process of freeze drying food. Under enough of a vacuum, the water molecules in ice will boil off, and the food is left dry, but with an airy crunch, since the food does not shrink as much as when it is dried above the freezing point, and when air is let back into the chamber, it fills up the cavities where the ice was.
Altitude
Not everyone has a vacuum chamber yet (although they are getting quite inexpensive these days). But another way to lower the air pressure is to travel up a mountain.
Cooking foods at high altitudes is different than cooking at sea level in several ways. Water boils at a lower temperature, so cooking rice or eggs in boiling water will take longer. Leavened baked goods have several problems at high altitudes. They rise faster, so yeast breads double in volume before they can get the same flavor from the yeast as they do at sea level. Cooks adjust for this by punching the dough down an extra time, and let it rise again.
Cakes also rise too fast, and then the bubbles pop, and the cake collapses. Cooks adjust for this by increasing the strength of the dough by adding more egg and more flour, and cook the cake at a higher temperature for a shorter time, so the dough sets before the bubbles pop.
Cakes also dry out faster at altitude, both because the low pressure allows water to evaporate more quickly, and because higher altitudes often have lower humidity than sea level locations. Cooks add a little more water to make up for this.
Adjusting the amount of leavening is also helpful, since a teaspoon of baking powder will make the same amount of gas as at sea level, but that gas will expand to a larger volume at altitude.
Shortening reduces the strength of the gluten molecules in the dough, so reducing the amount of shortening will help prevent the collapse of the bubbles. So will reducing the amount of sugar slightly, or replacing some of the sugar with nonfat powdered milk, which adds strength.
Raising the boiling point
Just as we can lower the boiling point, we can also raise the boiling point of water. Simply increasing the pressure works, and that is how pressure cookers work. The water boils and steam is created, but it can’t escape until it builds up enough pressure to lift the weight on the escape valve.
But we can also raise the boiling point by dissolving something in the water that does not itself boil (it is non-volatile). This works by diluting the water, so that fewer water molecules are at the surface, and thus fewer can escape. This effect is called reducing the vapor pressure of the water.
How much the substance raises the boiling point depends on how many particles of it (the solute) are dissolved in a given amount of water (the solvent). Some compounds break into more than one particle when they dissolve. Table salt breaks into a sodium ion and a chloride ion, so there are two particles. Sugar stays as one particle. Calcium chloride breaks into three particles – the calcium ion, and two chloride ions.
This comes into play when we try to calculate how much the boiling temperature will rise when we dissolve some amount of sugar or salt into the water.
Adding a teaspoon of salt to a quart of water (or 10 grams of salt to a liter of water) will raise the boiling point by 0.31° F (0.17° C). That small amount is not noticeable when cooking. To make a noticeable difference, the amount of salt would have to be so much as to make the food completely inedible. So adding salt to boiling water to make something cook faster just won’t work in practice.
As we have seen earlier, sugar reacts with water and with itself to make larger molecules when it is heated. Also, sugar melts at a temperature not that much higher than water. For these reasons, sugar raises the boiling point much faster than would be explained by merely diluting the water.
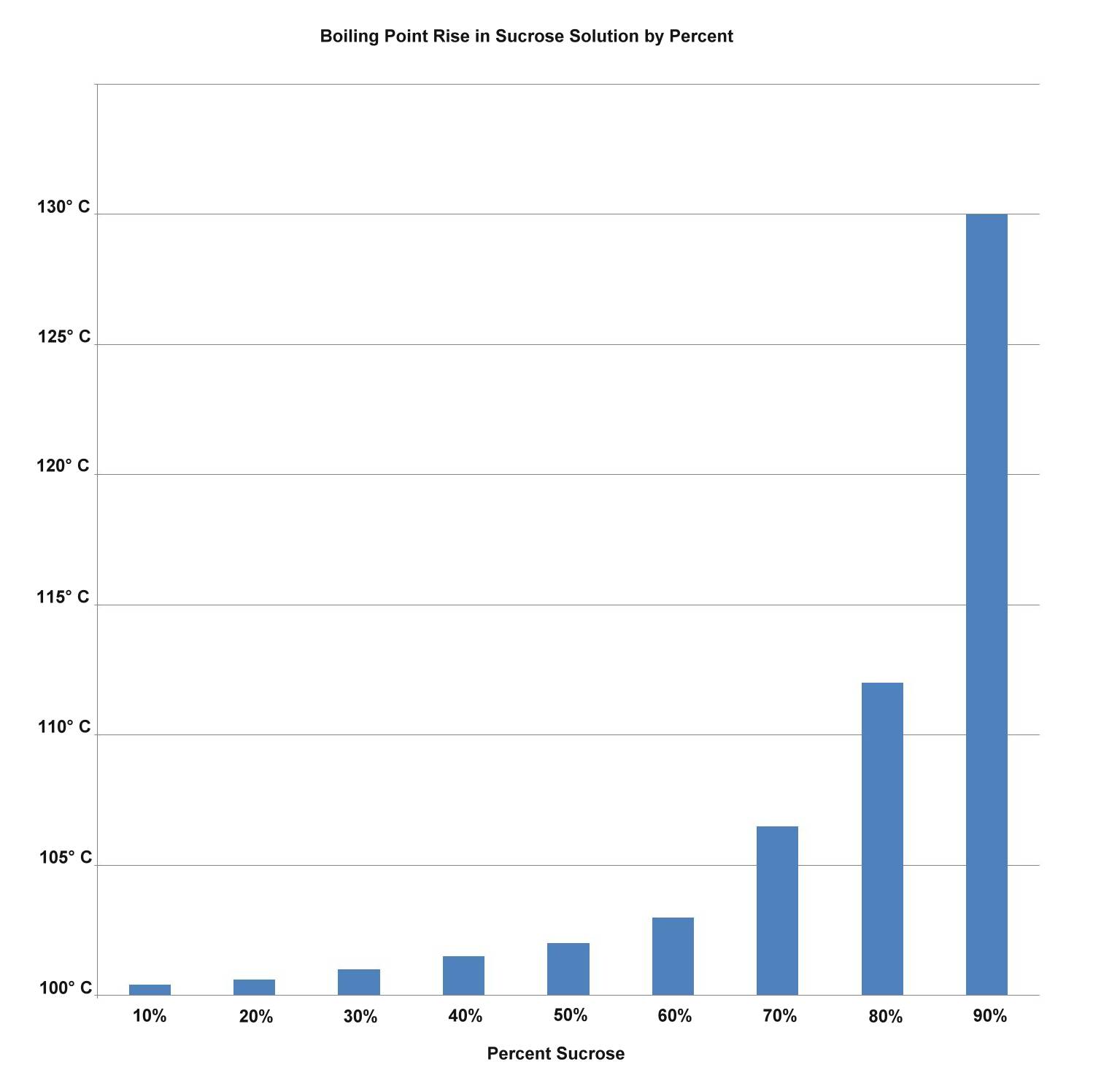
As the concentration of sucrose in the water increases, the amount of increase in the boiling point goes up even faster, unlike the linear graph salt would give. Some of the sugar molecules will break into glucose and fructose molecules. Fructose melts at 103° C, and some of the other sugars are going to be dissolved in liquid fructose, and so is some of the water. Things start to get complicated quickly.
Pressure cookers
In the kitchen, we use the boiling point of water as a convenient temperature modulator. Since the temperature cannot exceed boiling as long as there is water in the pot, we can let the pot boil and only worry about how long we leave the food to cook.
If we could choose a higher boiling point, we could cook food faster, and still only have to keep track of the time. A pressure cooker lets us do that.
Pressure cookers have several advantages over a pot of boiling water. The food is cooked at a higher temperature, so it cooks faster. When the pressure cooker is used to steam food (instead of boiling it), vitamins and minerals are kept in the food, and not destroyed by light or oxygen. The steam replaces the air in the pot, and the lid keeps the light out. And volatile flavor molecules are not lost into the air.
Some effects of cooking are due to temperature alone, and some are due to elevating the temperature for a length of time. Physical effects tend to be in the former category, such as softening tough connective tissue in meat, turning it into gelatin, or swelling the starch grains in vegetables to create a soft gel. Allowing more time in the pot favors the second category, where chemical reactions change the food, often destroying nutrients or producing bad flavors and odors. Quickly cooking food in a pressure cooker can get the benefits of temperature, without giving the food time to lose flavor and nutrition.
Some chemical changes produce flavors we like, and the main one that comes to mind is the Maillard reaction. But lucky for the cook, meat can be seared before being cooked under pressure, and we get the best of both.
Another advantage of pressure cookers is sterilization. The higher heat destroys microorganisms faster than they are killed in boiling water. When preserving foods by canning, heating the cans or jars in a pressure cooker kills harmful organisms in much less time.
Vacuum in canning jars
We have discussed high pressures, but low pressures are also available in the kitchen.
When we put food in a jar and cook it, water turns to steam in the jar, and this displaces the air. When we screw the top onto the jar while it is still hot, the steam is still there, keeping the air out of the jar.
But as the jar cools, the steam condenses back into water. This leaves a vacuum in the space where the steam was. The pressure of the air outside has not changed, and without steam pressure inside to hold it back, air pressure bends the metal lid down into the jar.
The lid is formed with a convex ridge so that as it is bent down into the jar, it snaps into a concave form. When we open the jar, air gets in, and the lid suddenly snaps back into its convex shape. The popping sound it makes lets us know that there was a vacuum inside the jar, and that bacteria have not been at work making gases that fill the vacuum.
Lowering the freezing point
Adding a solute to water raises the boiling point, but it also lowers the freezing point, and for the same reason. The freezing point is the temperature where as many water molecules are attaching to the ice (freezing) as there are water molecules leaving the ice (melting). If the temperature is above the freezing point, more water molecules will leave the ice than are joining it, and we get a puddle. If the temperature is below the freezing point, more water molecules with stick to the ice than are leaving it, and we get an ice cube.
Adding a solute like salt or sugar changes this equilibrium. The solute does not stick to the ice like a water molecule does. It merely gets in the way, taking the place of a water molecule that might have hit the ice and stuck. But it does not interfere with the water molecules that leave the ice. So more molecules leave the ice than freeze onto it. In order to restore the balance, we need to lower the temperature, until the same number of molecules are sticking as are leaving.
Making ice cream
We use salt to melt the ice on roads, although sugar or some other solute would also work. Salt is used because it is cheap. But if the temperature falls below -21° C, salt will no longer melt the ice, because it would require dissolving more salt than the water can hold (the water would be saturated with salt). The salt would just crystallize back out of the water, leaving a mixture of water, ice, and salt.
But melting ice has another useful property besides making highways less slippery. It makes things cold. It takes energy to break the bonds that hold water molecules together in ice. If we put an ice cube into room temperature water, the energy in the fast moving molecules in the water will get used up making chemical bonds as molecules freeze onto the ice. More molecules will leave the ice than freeze onto it, but the molecules leaving the ice will not have a lot of energy, and will be moving slower, and thus they will be colder.
The cooling effect continues, until the ice and the water reach their equilibrium temperature. For pure water, this is 0° C. But if we add salt or sugar, the cooling effect will continue until the temperature drops to the new equilibrium temperature. If we add a lot of salt, we will reach the point where no more will dissolve, and the equilibrium temperature will reach -21° C.
This is why we can freeze ice cream by putting it into a mixture of ice and salt.
